HomeResearchResearch Lines
Energy
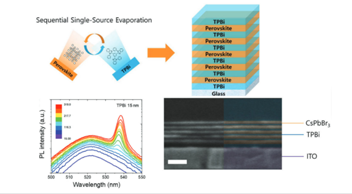
This transversal line involves most of the groups of the Institute, including the search of new materials for the production and storage of green Hydrogen, the development of batteries and supercapacitors, or new challenges in photovoltaic technologies. A holistic approach is being developed, addressing from multiscale theory to synthesis and development of devices covering a wide range of TRLs. One of the major driving forces is the technology transfer, which is reflected in the large number of patents and projects with private companies Research objectives and activities O6.1) Photo)electrocatalytic Water Splitting. Our experience in the development of molecular and 2D materials with a rational control over the chemical composition and supramolecular organization, will allow us to explore the effect of hybridization and/or the study of novel heterostructures that will be pivotal for the fabrication of novel energy materials with improved OER and HER catalytic activities. O6.2) New energy storage technologies. During the last 4 years we have developed new strategies for the preparation of lithium and sodium-ion batteries mainly based on 2D materials. Other family of compounds under study are the aqueous supercapacitors and the alkaline rechargeable batteries (Adv.Mater. 2019, 31, 1900189). We intend to develop novel devices based on our patented metal-collector free cells, that will revolutionize the electrode preparation either in aqueous (supercaps or alkaline batteries) or inert (Li and Na batteries) environments. O6.3) New decarbonization technologies. In the last 3 years we have developed new strategies in MOFs to facilitate CO2 capture without the limitations imposed by the current technology based on the use of amine solutions: on one hand, we have used ultramicroporous MOFs to enhance the CO2:N2 separation (J.Mater.Chem.A 2021, 9, 25189, J.Mater.Chem.A 2023, 11, 5320); on the other, we have integrated amines in the pores of MOFs (Angew.Chem.Int.Ed. 2024, 63, e202402973). We have also reported a hydrophobic MOF that selectively separates CO2 even under extremely humid conditions (ACS Appl.Mater.Inter. 2023, 15, 5309). We now plan to integrate capture with transformation by adequate modification of porosity metrics and chemical composition to use the captured CO2 as C1 feedstock for the formation of CO, CH4 or CH3OH by either thermal or photothermal catalysis (Chem.Catal. 2021, 1, 364). O6.4) Perovskite Photovoltaics (PVs): Perovskites-based nanomaterials and devices attracted significant attention during the last years (Angew.Chem. 2021, 60, 27312, J.Am.Chem.Soc. 2024 doi.org/10.1021/jacs.3c14335). This technology has demonstrated high efficiency (> 22%) in many device configurations, but concerns about stability and upscaling remain unclear. To date, high efficiencies are mostly obtained through non-scalable deposition methods using toxic environmental solvents, but less explored dry processes like vacuum deposition are emerging as eco-friendly and scalable alternatives. At present, PCE values over 21% have been demonstrated for vacuum deposited perovskites (24 % based on sequential evaporation). Still, these technologies are extremely slow for a real market integration. In addition, for commercial applications perovskite solar cells need to become stable (< 10% PCE lost at 85 °C after 1000 h). Understanding the degradation mechanisms in vacuum deposited perovskites, improving the stability, and developing faster deposition processes are clear challenges that we will target in the next years.